Studying how the plasmacytoid dendritic cells sense and control viral infection.
Responsable de l’encadrement : Dreux Marlene
Tél : 06 45 42 20 41 E-mail: marlene.dreux@ens-lyon.fr
ENS Lyon
Résumé du Projet de Stage
All cells can detect infection by viruses and trigger an innate immune response. This first line of defense is initiated by the recognition of viral elements by host receptors. This detection leads to the production of antiviral molecules, including type I and III (λ) interferons (herein referred to as IFN-I/λ). IFN-I/λ are essential response to initiate the immunity against viral infections.
The plasmacytoid dendritic cell (pDC) is a unique immune cell type specialized for rapid and massive production of IFNI/λ. The main viral-sensors responsible for pDC activation are the Toll-like receptors, which recognize viral genomes. Studies have demonstrated that pDCs migrate into infected tissues. The rapid production of IFN-I/λ by pDCs is essential to control various viral infections, as we previously uncovered1,2. Importantly, we have demonstrated that pDCs establish cell contact with infected cells that sustains their robust antiviral responses3. This is mediated by molecular reorganizations at the contact site between pDCs and infected cells, including a specific polarization of actin network and the endocytosis machinery3. Thus, we uncovered a specialized platform for PAMP transfer from infected cell to the host sensor in pDCs. The detection by pDC then leads to IFN-I/λ production and an antiviral response. This antiviral signaling also acts as positive feed-forward loop culminating by promoting polarization and sustained contact of pDCs with infected cells3. Therefore, we discovered a new type of structure that we named the interferogenic synapse (iSYN)3. The requirement for this structure is now demonstrated for genetically-distinct viruses2,3. This specialized ability of pDCs to sense infected cells brings key questions on how pDC achieve an optimal antiviral response against viral infection at the site of infection, while minimizing the deleterious impact of hyperactive inflammation. In addition, our recent results revealed that iSYN is also a structural platform for polarization of effector functions leading to targeted IFN-I/λ secretion directly toward the eliciting infected cells.
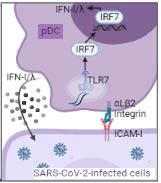
Model of the innate responses against viruses. The short-range sensing of virus-infected cells (at the bottom) by pDCs (at the top) requires cell contact mediated by adhesion complexes (identified as αLβ2 integrin and ICAM-1). This triggers a TLR7-induced signaling leading to an IFN-I/λ- prioritized response, while leaving inactive the NF-κB-mediated signaling.
Objectives: Define the dynamics and the molecular events at iSYN leading to pDC
antiviral response.
1. How is the antiviral secretion of pDCs targeted towards the infected cells?
2. What are the dynamics of iSYN formation duration, strength and renewal?
Therefore, this project is designed to functionally dissect the molecular basis of the targeted antiviral response
by iSYN. Additionally, our studies shall provide mechanistic insights on the largely still-unknown secretion process of
cytokines, including IFN-I/λ and other pro-inflammatory cytokines.
Methods: To address these questions, we have already established a methodology to monitor at the single-cell level the
impact of pDC response on virus-infected cells by cutting-edge imaging technology (i.e., in live confocal microscopy,
ImageStream X)2-5 and novel functional model to study the dynamics and regulations of pDC antiviral response.
Novelty of the project: The strength of this project is to answer questions on the host/pathogen interactions by
combining approaches from disparate fields: cellular biology (live-imaging microscopy), molecular biology and
immunology (antiviral response: RT-qPCR, ELISA, Western blot) and virology (viral replication: immunofluorescence, RTqPCR).
The studied viruses are especially Hepatitis E virus, a pathogenic virus now considered as an emerging problem in
industrialized countries and SARS-CoV-2, already studied in our lab.
References
1 Webster, B. et al. Plasmacytoid dendritic cells control dengue and Chikungunya virus infections via IRF7-regulated interferon responses. Elife 2018, 7
2 Venet, M. et al. Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nat Commun 2023, 14, 694.
3 Assil, S. et al. Plasmacytoid Dendritic Cells and Infected Cells Form an Interferogenic Synapse Required for Antiviral Responses. Cell Host Microbe 2019, 25, 730-745 e736.
4 Coleon, S., Assil, S. & Dreux, M. Monitoring of Interferon Response Triggered by Cells Infected by Hepatitis C Virus or Other Viruses Upon Cell-Cell Contact. Methods Mol Biol 2019, 1911, 319-335.
5 Assil, S. et al. Sensing of cell-associated HTLV by plasmacytoid dendritic cells is regulated by dense beta-galactoside glycosylation. PLoS Pathog 2019, 15, e1007589.
Dernières Publications en lien avec le projet :
- Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Venet
M, Ribeiro MS, Décembre E, Bellomo A, Joshi G, Nuovo C, Villard M, Cluet D, Perret M, Pescamona R,
Paidassi H, Walzer T, Allatif O, Belot A, Trouillet-Assant S, Ricci EP, Dreux M. Nature Communication 14,
694 (2023). https://doi.org/10.1038/s41467-023-36140-9. - Adaptation to host cell environment during experimental evolution of Zika virus. Grass, V., Hardy, E.,
Kobert, K. Rastgou S.T., Décembre E, Guy C, Markov P.V.., Kohl A., Paris M., Böckmann A., Muñoz-González
S., Sherry L. , Höfer T., Boussau B. & Dreux M. Commun. Biol. 5, 1115 (2022). https://doiorg/
10.1038/s42003-022-03902-y. - Interplay between SARS-CoV-2 and the type I interferon response. Sa Ribeiro M, Jouvenet N*, Dreux M*,
Nisole S*. PLoS Pathogens. 2020 Jul 29;16(7):e1008737. doi: 10.1371/journal.ppat.1008737. eCollection
2020 Jul. PMID: 32726355. *co-last authors. - Plasmacytoid Dendritic Cells and Infected Cells Form an Interferogenic Synapse Required for Antiviral
Responses. Assil S*, Coléon S*, Dong C, Décembre D, Sherry L, Allatif O, Webster B & Dreux M. Cell Host
and Microbe. 2019. Apr 15; 25(5):730-745.e6. doi: 10.1016/j.chom.2019.03.005 - Sensing of Cell-associated HTLV by Plasmacytoid Dendritic Cells is Regulated by Dense-Galactoside
Glycosylation. Assil S, Futsch N, Décembre E, Alais S, Gessain A, Cosset FL, Mahieux R, Dreux M* &
Dutartre H*. PLoS Pathogens. 2019. Feb 28;15(2):e1007589. doi: 10.1371/journal.ppat.1007589. * co-last
authors. - Plasmacytoid dendritic cells control dengue and Chikungunya virus infections via IRF7-regulated interferon
responses. Webster B, Werneke SW, Zafirova B, This S, Coléon S, Décembre E, Paidassi H, Bouvier I,
Joubert PE, Duffy D, Walzer T, Albert ML, Dreux M. Elife. 2018 Jun 19;7. pii: e34273. doi:
10.7554/eLife.34273.
Website: https://ciri.ens-lyon.fr/teams/VIV
Ce projet s’inscrit dans la perspective d’une thèse
Ecole Doctorale de rattachement : ED ENS Lyon – BMIC
Equipe d’Accueil :
Intitulé de l’Unité : CIRI, INSERM U111, ENS Lyon.
Nom du Responsable de l’Unité : François-Loic Cosset
Nom du Responsable de l’Équipe : Dreux Marlene
Adresse : ENS Lyon, 46 allée d’Italie, 69007 Lyon
https://ciri.ens-lyon.fr/teams/VIV

